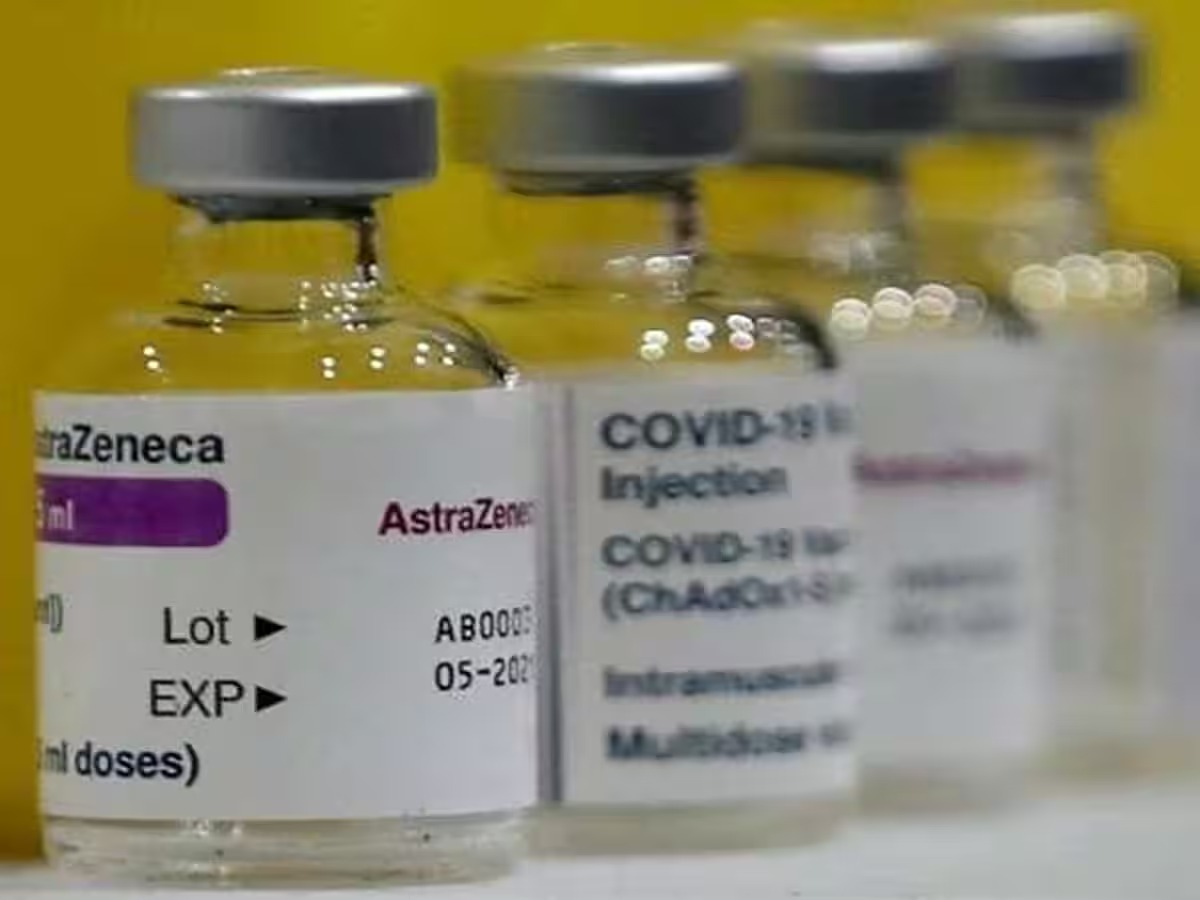
AstraZeneca Latest News: AstraZeneca (AZN Limited), the company making 'Covishield' (Covid-19), will withdraw its corona vaccine from across the world. On Tuesday (7 May 2024), the British multinational pharmaceutical and biotechnology company of Swedish origin said that it has started the process of recalling the vaccine. In the report of news agency 'Reuters', it has been further alleged in the company's allegation that it had to take this step due to lack of demand.
AZN Ltd has informed that it will proceed to withdraw the marketing authorization of Vaxzeveria vaccine in Europe. According to the company's statement, many Covid 19 vaccines have been developed after the Corona epidemic. Updated vaccines are also available in the markets. AstraZeneca also said demand for its Vaccinia vaccine had fallen because there were too many vaccines already on the market. That is why it is neither being manufactured nor supplied.
Potential risk of blood clots – company admitted in court,
The move by the maker of the COVID-19 vaccine comes days after AstraZeneca admitted for the first time in court documents that its vaccine, developed in partnership with Oxford University, could cause a rare and dangerous condition. There is a serious risk of blood clotting, however, on reports of confusion regarding the vaccine, health experts say that the benefits of the vaccine are more and the disadvantages are less. In such a situation, there is no need to panic about the Covishield vaccine. The vaccine is safe and the side effects that were expected occurred only after vaccination.
AstraZeneca's vaccine, known as Covishield in India,
Oxford-AstraZeneca's Covid vaccine, sold as Covishield in India and Vaxjavria in Europe, is a viral vector vaccine developed using a modified chimpanzee adenovirus. Covishield, manufactured and marketed in Hindustan in partnership with the Serum Institute of India (SII), was widely administered in India to about 90% of the Indian population.
 look news india
look news india